Mission: The mission of the Proof of Concept Office (POC) is to empower Cleveland Clinic caregivers to transform promising research and novel ideas into medical products that significantly advance human health. The POC Office collaborates with Cleveland Clinic Innovations (CCI) and directly with inventors to identify projects stemming from new invention disclosures that promise clinical benefits, potential for intellectual property, and commercial viability. These projects may require assistance with prototyping or initial demonstrations. Before filing an invention disclosure, inventors seeking to develop compelling medical products and explore funding opportunities for product development are encouraged to consult with the POC Office.



Ideas are referred to the POC Office from Cleveland Clinic Innovations (CCI) or through engagements directly from inventors. POC projects come from new invention disclosures that can make a positive clinical impact and show an opportunity for new intellectual property and commercial outcomes but need help with prototyping or initial demonstration. Inventors can also approach the POC Office before filing an invention disclosure, for guidance on creating a compelling new medical product and to learn how to access product development funding.
Our Team
Bryan Williams, PhD
Scientific Advisor
Elizabeth Sump
Senior Director,
Strategic Alliances
James D. Ellis, JD, MBA
Managing Director,
Pre-Market Innovations
Catherine Teringo, MHA
Program Manager
Julie Woda, PhD
Senior Director & Domain Lead,
Therapeutics & Diagnostics
Vara Prasad Josyula, PhD
Senior Director,
Therapeutics & Diagnostics
Erik Waldorff, PhD, MBA
Senior Director,
Medical Devices
Snehal Chokhandre
Senior Principal Research Engineer Biomedical Engineering, Senior Project Manager for Proof of Concept

Therapeutics
PAI-1 Inhibitors as Therapeutics and Diagnostic Indicators
Inventors: Thad Stappenbeck, MD PhD; Vara Prasad Josyula, PhD
Mobius Care, Inc., a CCI portfolio company, uses PAI-1 inhibitors to personalize Inflammatory Bowel Disease (IBD) therapeutic and diagnostic care, through its proprietary AI tool, applied to targeting host and microbiome pathways that infect gastrointestinal (GI) tract wounds. Vara Prasad Josyula PhD and Juliana Woda PhD from the POC team worked with the inventors to define a business model and growth plan to help make Mobius Care a new CCI startup company. The Company’s technology can also identify subsets of patients who are likely to respond favorably to an existing arsenal of well-characterized drug candidates. Additionally, Mobius is developing therapeutics to treat the dysfunctional wound healing and dysfunctional microbiome associated with IBD.
-1-1.png?width=1200&length=1200&name=Untitled%20design%20(15)-1-1.png)
Devices
Autoregistration for Stereotactic Procedures
Inventors: Rafi Avitsian, MD; Pablo Recinos, MD
This invention disclosure was originally disqualified due to a lack of invention enablement. Dr. Erik Waldorff worked with the Inventors in a series of Ideation Clinics, resulting in a new invention disclosure and a SPARK award. The funding was used for enablement and to define features. The new Invention was admitted into the Innovation’s system less than 10 months later, a patent was filed for the new invention, and a Joint Development Agreement was established with an external partner to help further enable the invention.
-1.png?width=1200&length=1200&name=POC%20(2)-1.png)
Devices
Modular Needle Valve for Injection Flow Control
Inventor: Michael Forney, MD
This initial Invention Disclosure arrived with few details about implementation or product features. To define the product features, the POC team conducted Ideation Clinics, voice-of-the-customer sessions, and prototyping experiments. This led to intellectual property filing, and the invention was successfully transitioned to CCI for active development of a minimal viable product.
-1-1.jpg?width=1200&length=1200&name=Jan%20Jul%20(2)-1-1.jpg)
Temporally-Corrected Dose Accumulation (Jacob Scott, MD)
This invention disclosure arrived lacking a proof-of-concept demonstration and details regarding the algorithms needed for enablement. The POC team and Innovation Fellows worked with the inventor to define critical path experiments, which resulted in a white paper defining the algorithms and subsequent invention qualification.
Ideation Clinics
An Ideation Clinic includes a panel of field experts from the POC team, Innovations, Medical Device Services and the inventor’s institute(s). who provide iterative constructive feedback to Cleveland Clinic medical device inventors to refine their ideas and arrive at well-founded inventions. Ideation Clinics facilitate two-way discussions of commercialization roadblocks and potential effects through considerations including:
- Technical feasibility and engineering- Reduction-to-practice
- Regulatory
- Clinical evidence
- Reimbursement
- Market Adoption

Invention Support/Idea-2-Invention (I-2-I)
Working with CCI Engagement Partners and Institute Innovations Leads, the POC team helps new inventors better understand the technology development process from "Ideas-to-Inventions (I-2-I)." Using the inventor’s great new ideas as the starting point, the POC team and Innovation Fellows help inventors understand the market, define objectives and value propositions, plan next steps, and identify internal and external development partners and sources of funding. This support enables inventors to develop "great ideas" into "great inventions."

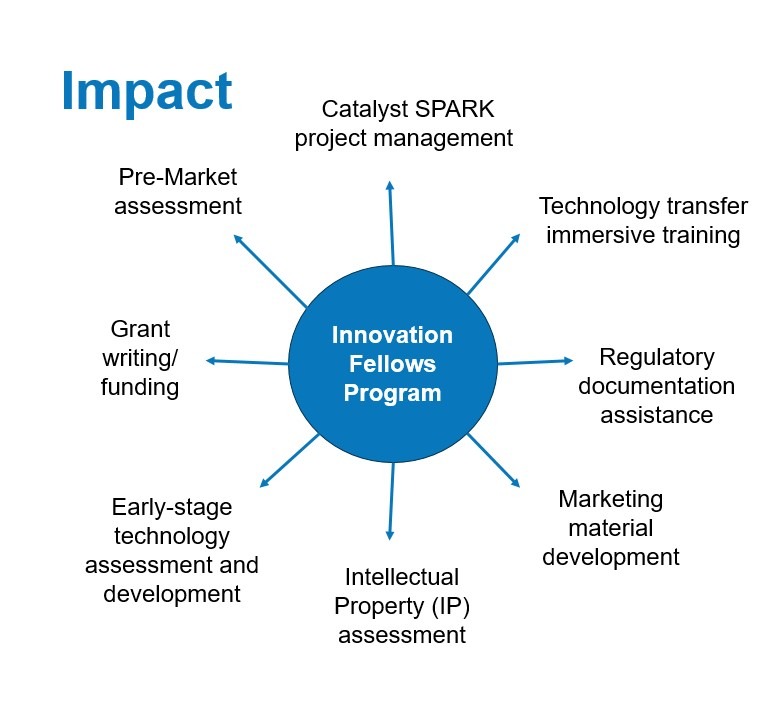
Innovation Fellows Program
Since 2018, the Innovation Fellows program has trained Cleveland Clinic caregivers in the healthcare technology development and commercialization process, from invention-creation through regulatory approval and marketing. It is a 2-year program and requires a 20% time commitment (with salary offset). Innovation Fellows receive didactic training and hands-on project management and, in their second year, they help train the incoming class. Fellows manage I-2-I and Catalyst SPARK projects. Graduates have gone on to new careers in CCI, joined Cleveland Clinic portfolio companies, become consultants, and have roles in industry. Fellows moving on to industry often facilitate collaborative partnerships between their company and Cleveland Clinic.

Bridging the gap between translational research and new therapeutics, Cleveland Clinic’s Center for Therapeutics Discovery (C3TD) was established in 2018 work with investigators to de-risk promising approaches using small molecule chemical probes. Part of an advanced research technology facility housed in Lerner Research Institute, C3TD includes a state-of-the-art small molecule screening library, X-ray crystallography, computational tools, and target validation to generate starting points for nascent hit-to-lead and lead optimization campaigns. Staff members comprise structural biologists, medicinal chemists, and pharmacologists. In addition to guiding and collaborating on projects that are referred by Cleveland Clinic Innovations, the POC team helps researchers interact with the C3TD as they are planning their drug discovery programs. C3TD also works with POC to secure and optimally utilize contract research organization (CRO) resources.



Medical Device Solutions
Medical Device Solutions (MDS) develops innovative prototypes and equipment to enhance research and solve unmet clinical needs. The MDS team includes experts in 3D printing, bio-robotics, mechanical testing, electronics, engineering design, instrument refurbishing and repair, prototyping, nitinol, and polymer fabrication. MDS team members participated as panel experts in POC Ideation Clinics.
In 2023, POC and MDS implemented a project tracking system and collaborated on 15+ projects including:
- Medical device design iterations
- Enablement planning
- Proof-of-concept prototype developments
.png)

Strategic Fundraising Support
To accelerate the inventor’s research and development activities, the POC team and Innovation Fellows assist in developing proposals for funding from public and private programs including the following:
- National Institute of Health (NIH)
- State of Ohio development
- Foundation and Philanthropic
- Small Business Innovation Research (SBIR)
- Small Business Technology Transfer (STTR)
Catalyst SPARK
In 2023, Cleveland Clinic’s Philanthropy Institute launched Catalyst SPARK, a part of the Caregiver Catalyst Grant Program. This program equips new inventors with strategic funding, effective project management, mentoring, and inventor education to help enable new technologies. The POC team helps validate and develop inventions selected for the program, including proof of concept, demonstration and prototyping. Participating inventors are partnered with a POC field expert, who guides the inventor through “Idea to Invention” milestones. Successful new inventions are then transitioned to Cleveland Clinic Innovations (CCI) for further development and commercialization.

.jpg)
.png)
Strategic Partnerships
The POC team works with external partners to provide resources to support research and development. Our objective is to combine the resources and talents of the partners to deliver mutually beneficial outcomes greater than what either partner could produce alone. Highlights from 2023 include: Moderna
- $1.6M educational partnership
- Emerging Research co-development
ThermoFisher
- Consulting and Equipment for Cleveland Clinic Cell Therapy Facility
- Exploring diagnostics research & development opportunities
Emerging/expanding relationships with pharmaceutical, medical devices, and materials companies
Cleveland Clinic Ventures Support
The POC team collaborates with Cleveland Clinic Innovations Ventures to support Cleveland Clinic SBIR-funded startup companies with company formation, board participation, and strategic fundraising.




.png?width=1200&height=1500&name=Untitled%20design%20(19).png)




























